The periodic table, the brainchild of Russian chemist Dmitri Mendeleev and published 150 years ago, is one of the most famous symbols of chemistry and can be found in classrooms all around the world. However, the current chart’s solid squares and familiar patterns hide one of its key characteristics: it is not “the” periodic table.
It has always been open to change. It has not only expanded as more elements have been found, but it has also added columns and undergone structural changes as our knowledge of the elements’ qualities and their interrelationships has expanded. And its best setup is still up for debate amongst scientists.
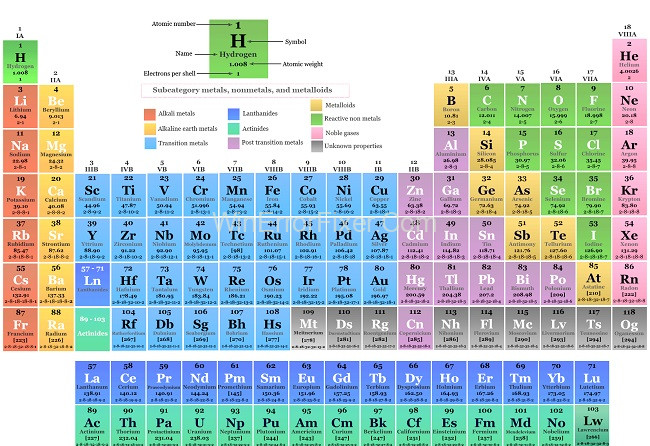
It has been argued that the elements’ chemical characteristics ought to determine their positions in the periodic table. Others argue that a more fundamental principle, such as electrical configuration or atomic number, is required. Parties disagree on how many rows the periodic chart should have, where helium should go, and which elements belong in group 3.
They are the latest in a long line of chemists and physicists who have attempted to bring some order out of the chaos that is the periodic table.
Michael D. Gordin, a historian at Princeton University who has written about Mendeleev, Julius Lothar Meyer, and other early creators of periodic tables, says, “What I find remarkable about the current argument is that there are those who insist on their being one right table.”
“People like Mendeleev and Lothar Meyer would have thought it was strange.” According to Gordin, the pioneers of the periodic table knew that different tables may represent natural laws in different ways, yet they still believed their tables were a mirror of natural laws.
For those of us who have become accustomed to seeing the familiar shape of the table on everything from coffee mugs to bathroom curtains, this may be a bit of a stretch to conceptualise.
Elements Arranged On The Periodic Table
Not only was Mendeleev not the first to see patterns in the elements, but he also wasn’t the first to try to represent those patterns visually. In 1829, for example, chemist Johann Wolfgang Döbereiner found three elements that had certain qualities. These are now understood to be chemical elements that belong to the same group, such as chlorine, bromine, and iodine.
In 1862, geologist Alexandre-Émile Béguyer de Chancourtois presented a cylinder with the elements spiralling up it based on their atomic weight, which he called a “periodic table.” The items in each column were similar.
In 1869, Mendeleev released his periodic table (which he dubbed a “periodic system”). It contained all 56 elements at the time and, if you squint, it looks a lot like the modern periodic table, only flipped on its side by 90 degrees.
In Mendeleev’s periodic table, each element is placed in a row according to its atomic weight, and each row is further subdivided into columns based on the number of other atoms it may combine with and other shared qualities.
Because of his confidence in the periodic system’s ability to anticipate both the existence and attributes of as-yet-undiscoverable elements, Mendeleev provided spaces in his table for these hypothetical elements. Mendeleev beat Lothar Meyer to publishing of a nearly identical table by a few months, ensuring the Russian’s position in history.
The development of quantum physics and atomic numbers laid the groundwork for the current debates over the periodic table. Although Mendeleev’s table is theoretically arranged from heaviest to lightest atom, the chemical characteristics of each element ultimately swayed his decision.
Mendeleev ranked tellurium higher than iodine, despite the former element being somewhat heavier, since it shares the same valence as oxygen, sulphur, and the other elements in its group.
It’s still the same arrangement in the tables. The fact that tellurium contains one fewer proton than iodine, and is hence one atomic number less, explains why each element is placed where it is. This fact was unknown to Mendeleev. According to Gordin, the periodic table “gains logic” once atomic numbers are known.
The discovery of electrons and the concept of atomic orbitals from quantum mechanics followed shortly after the discovery of protons.
These results offered a fresh perspective on the logic behind the periodic table. The structure of Mendeleev’s system remained unchanged, but scientists realised that the electronic structure of an element was the primary factor in determining its qualities and the reason why elements in the same group shared those traits.
The Madelung rule, also known as the aufbau principle, explains why electrons fill the 1s orbital before moving on to the 2s and 2p orbitals, and so on down the periodic table. That’s how we got to the modern tables, which aren’t drastically different from the ones renowned chemist Glenn T. Seaborg drew in the 1940s.
Seaborg removed the f-block elements, also known as the lanthanide and actinide series, from the main table and left them to float below. This choice is often seen as a compromise for practicality, as if all the columns and rows were the same size, the table wouldn’t fit on a regular sheet of paper and the type would have to be too small to read.
To make up his f-block, Seaborg used no less than fifteen different components. From an electrical configuration perspective, this makes little sense because the f orbitals can only house a maximum of 14 electrons.
However, many tables have this characteristic, including the table on the website of the International Union of Pure and Applied Chemistry (IUPAC), which is the final authority on naming elements and compounds. This allows us to sidestep one of the most divisive issues surrounding the periodic table.
Assemblage 3: which elements go here? Absolutely everyone agrees that yttrium and scandium are important. What, then, are the elements that come after those two? A combination of lanthanum and actinium? Alternatively, you might use lutetium and lawrencium.
Groups
The periodic tables currently used in textbooks, laboratories, and lecture halls are not standardised in any way. While some opt for an f-block with 15 elements to sidestep the group 3 issue. Some people have La and Ac in group 3, while others have Lu and Lr in that spot with the rest of the f-block down below.
IUPAC has formed a committee to settle the matter once and for all. According to IUPAC’s website, one reason for organising the task force was to end debates between educators and students over which table should be used in the classroom.
According to science journalist and working group participant Philip Ball, the issue boils down to whether or not the table is shaped by physics or chemistry. His explanation boils down to this: the group is discussing whether to put more stock in the chemical behaviour of elements or in the quantum physics that dictates their electronic configurations.
An advocate for chemical behaviour is Guillermo Restrepo, a mathematical chemist at the Max Planck Institute for Mathematics in the Sciences. Restrepo bases the proper arrangement of the chairs on past practises.
He mentions how Mendeleev and his contemporaries discovered periodic systems by analysing the properties of elements, particularly the binding behaviour of certain elements. According to Restrepo, “in the heart of the periodic system, what you have is chemistry, and you require chemical reactions.”
To learn how chemical processes contribute to the periodic system, Restrepo and coworkers investigated over 4,700 binary compounds including 94 elements (MATCH Commun. Math. Comput. Chem.2012, 68,417). More than one atom, but no more than two, might make up the molecules.
The scientists devised a chart that puts chemically related elements close together. Fluorine, chlorine, and the other halogens, for instance, cluster together because they share chemical and physical properties with many other elements.
According to Restrepo, the similarities between lanthanum, scandium, and yttrium are greater than those between lutetium and any of the other elements in group 3. However, the approach does not yield a satisfying conclusion on Lr versus Ac. According to Restrepo, the lack of information about Lr and Ac’s binding to other entities is the root of the problem.
Ac gives just about 70 data points, and Lr, fewer than 40, although there are tens of thousands of compounds one may utilise to analyse the similarities of Sc, Y, La, and Lu, as stated by Restrepo.
Chair of the IUPAC working committee and philosopher of science at the University of California, Los Angeles Eric Scerri disagrees. Sc, Y, Lu, and Lr are his proposed group 3 elements. Scerri believes that emphasising only chemical or physical characteristics is a mistake. He likens it to the way early botanists categorised flowers based on colour or number of petals.
Scerri advises aiming towards the basics, citing electronic configuration as an example. Having a large number of properties isn’t the answer, and that’s why you shouldn’t pursue it.
Scerri will tell you that the electronic setup isn’t flawless either. Some elements, such as copper, have been given special consideration for the filling of their orbitals. All elements in group d of the periodic table should have their s orbitals filled, according to the table.
On the other hand, copper defies reason. For electron configuration, it should be [Ar] 3d9 4s2. Rather, it adopts the more stable configuration [Ar] 3d10 4s1, where one electron occupies the empty 4s atomic orbital and nine occupy the 3d shell.
Scerri does favour electronic setup above his “gross physical qualities” for tabular organisation. But there is a much more straightforward way, in his view, to handle the group 3 issue; just sort the elements by their atomic numbers. We will all have to adjust to Scerri’s proposed seating arrangement if he has his way.
Expanding Outwards
What I propose is this,” Scerri says. Create a 32-column representation of the periodic table.
Scerri claims that the current dominance of the 18-column version of the periodic table is due solely to convenience, and that the 32-column version is the more natural. You can utilise the atomic numbers to build a 32-column table.
Scerri argues that 32 columns is more accurate since it returns the f-block to its proper location within the table, rather than having it float below for convenience, which would address the group 3 problem.
Lanthanum (atomic number 57) follows barium (atomic number 56) to initiate the f-block, with actinium below it in a 32-column table ordered by rising atomic number.
Lutetium is the first member of group 3, which includes scandium, yttrium, and lawrencium. Scerri’s need for a primary organising principle is fulfilled by the strict devotion to atomic number, which also neatly avoids any inquiries into the elements’ chemical or physical qualities.
How Are the Elements on the Periodic Table Arranged?
The elements in the Periodic Table are arranged based on their atomic number, which is the number of protons present in an atom of that element. This layout starts from Hydrogen, with an atomic number of 1, and goes up to elements with ever-increasing atomic numbers. The rows are called “periods” and the columns are referred to as “groups.”
What Tells How Elements Are Arranged in Order?
Atomic Number:
As mentioned, the atomic number primarily governs the arrangement of elements. Elements in ascending atomic numbers have incremental numbers of protons and usually electrons.
Electron Configuration:
Another pivotal factor is the electron configuration of the atoms. Elements in the same column (group) often have similar electron configurations, which leads to similar chemical properties.
Chemical Properties:
Groups often consist of elements that behave similarly in chemical reactions due to their similar electron configurations. For instance, the noble gases, found in Group 18, are all odorless, colorless, and inert.
How Are the Elements Arranged in the Periodic Table Quora-Style Explanation:
If you’re a fan of simplified explanations, think of the Periodic Table like a library. Just as books are arranged based on genres, languages, or authors, elements are categorized by atomic numbers and similar chemical properties. This makes it easy to predict how a certain element will behave under specific conditions.
Why Is the Arrangement Important?
Predictive Tool:
One of the greatest utilities of the Periodic Table is its ability to predict element behavior. Knowing where an element is positioned can offer a wealth of information about its properties.
Scientific Understanding:
Understanding the arrangement helps in grasping other scientific concepts, including atomic structure, electron configuration, and even quantum mechanics to some extent.
Practical Applications:
From creating new materials to drug discovery, the structured arrangement helps scientists and researchers in various fields of scientific inquiry.
Conclusion
The Periodic Table is not just a collection of elements, but a well-thought-out system that gives us insight into the behavior of elements and compounds.
The atomic number, electron configuration, and chemical properties are key factors that dictate this arrangement. This organization is crucial for educational, predictive, and practical purposes, making the Periodic Table an indispensable tool in the realm of science.



















